Widefield microscopy
Equipment
- Equipment
EVOS
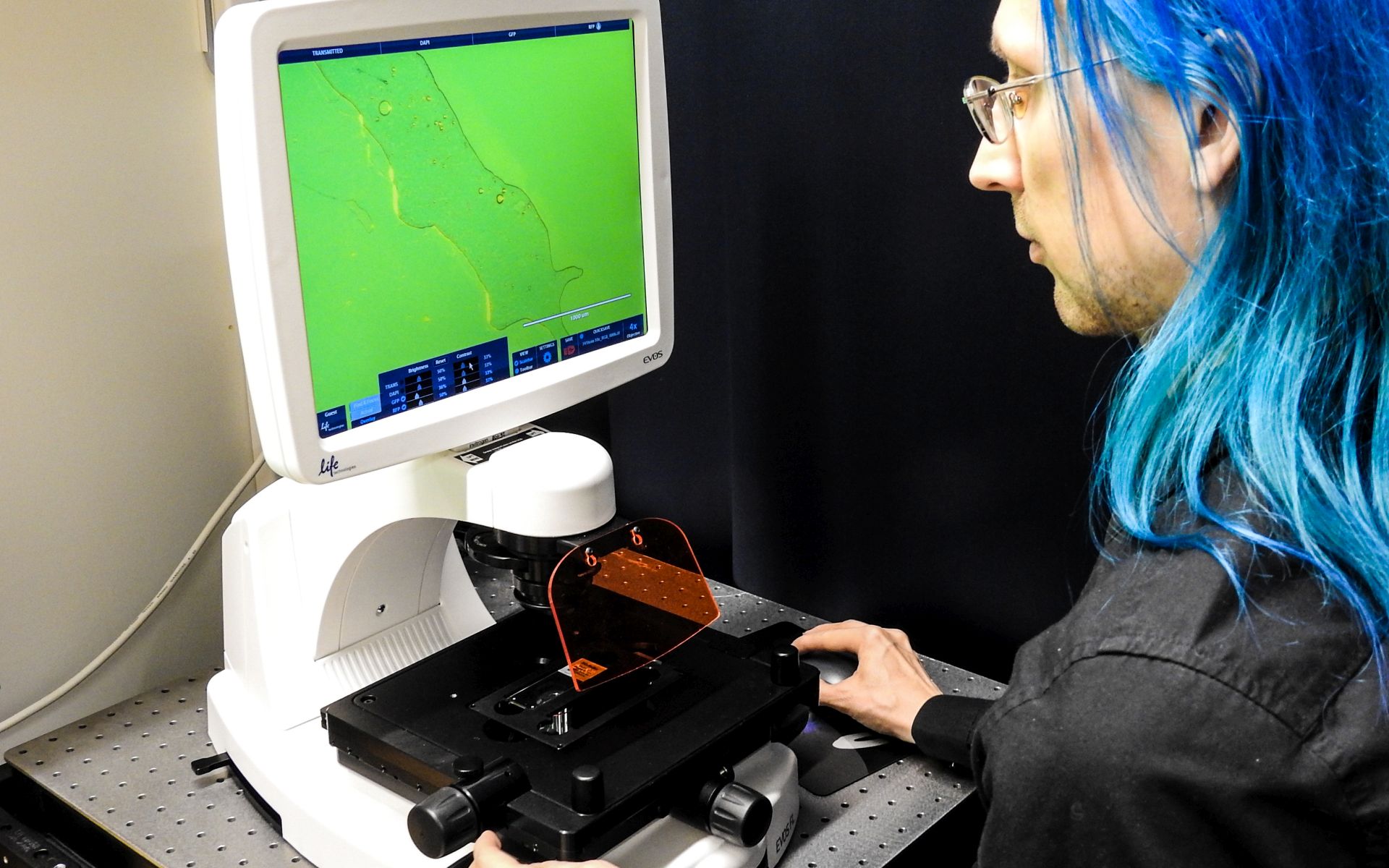
Inverted epifluorescence microscope with long working distance objectives for bright-field, phase-contrast, and fluorescence microscopy.
Mainly used for cells growing on plastic bottom dishes. Image acquisition through integrated camera and software, images stored directly to a memory stick.
NB! Use the USB port on the microscope. The port on the screen is not connected to it.
Technical Information and capabilities
Objectives
Magnification | Objective type | NA | Working distance (mm) | Phase-contrast | Immersion |
|---|---|---|---|---|---|
4X | LPlan Fluor | 0.13 | 16.9 | Ph | Dry |
10X | Plan Fluor | 0.30 | 8.3 | - | Dry |
20X | Plan Fluor | 0.45 | 7.1 | - | Dry |
40X | Plan Fluor | 0.65 | 2.8 | - | Dry |
Filters
Position | Light Cube | Excitation (nm) | Emission (nm) |
|---|---|---|---|
1 | Transmitted light | - | - |
2 | DAPI | 357/44 | 447/60 |
3 | GFP | 470/22 | 510/42 |
spare | RFP | 531/44 | 593/40 |
4 | TxRed | 585/29 | 624/40 |
Fluorescence filters are integrated with LED light sources into 'light cubes'. The system has space for 4 such cubes or 3 + transmitted light.
Camera / Accessories
Integrated monochrome CCD camera.
An assortment of sample holders for multi-well plates, dishes, flasks, and microscope slides.
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.
https://www.fpbase.org/microscope/KXH56NF8YtWFimM9D9Km5f/
Key Words
Wide-field | Inverted microscope | Long working distance |
|---|
Zeiss Axio Imager 1

Motorized upright epifluorescence microscopes with transmitted light (bright field, phase contrast and dark field) and a motorized stage.
Technical Information and capabilities
Objectives
Magnification | Objective Type | NA | Working distance (mm) | Phase-contrast | Immersion |
|---|---|---|---|---|---|
5x | EC Plan Neofluar | 0.16 | 18.5 | Ph1 | Dry |
10x | EC Plan Neofluar | 0.3 | 5.2 | Ph1 | Dry |
20x | Plan Apochromat | 0.8 | 0.55 | Ph2 | Dry |
40x | EC Plan Neofluar | 0.75 | 0.71 | - | Dry |
40x | EC Plan Neofluar | 1.3 | 0.21 | - | Oil |
63x | Plan Apochromat | 1.4 | 0.19 | - | Oil |
100x | EC Plan Neofluar | 1.3 | 0.20 | - | Oil |
Filters
Position | Filter | Excitation | Emission | Dichroic |
|---|---|---|---|---|
1 | Transmitted light | - | - | - |
2 | 49 DAPI | 365 | 445/50 | 395 |
3 | 47HE CFP | 436/25 | 480/40 | 455 |
4 | 38HE GFP | 470/40 | 525/50 | 495 |
5 | 46HE YFP | 500/25 | 535/30 | 515 |
6 | 31 Alexa 568 | 565/30 | 620/60 | 585 |
7 | 64HE mPlum | 587/25 | 647/70 | 605 |
8 | 50 Cy5 | 640/30 | 690/50 | 660 |
9 | 25HE DAPI/FITC/TxRed | Triple | Triple | Triple |
10 | Cy7 | 708/75 | 810/80 | 757 |
Camera
- Hamamatsu Orca Flash 4.0 LT B&W camera for fluorescence imaging.
- Zeiss AxioCam 105 color camera for transmitted light imaging.
Lightsource
- HXP 120 V fluorescence light source with motorized brightness adjustment and integrated shutter.
Software
- Zeiss Zen 2.
User manuals and quick instructions:
There are new Quick Start Guides available for ZEN Blue. They include the "First Steps", multidimensional imaging and import & export guides.
Some of the functions and images refer to the ZEN Lite and are different from the full version we have, which has more functionality.
There is also a video tutorial available on YouTube.
How to set up the Köhler illumination video tutorial on YouTube.
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.
https://www.fpbase.org/microscope/5WghnCQQbmeV3sED2RmZL4/?d=hamamatsu-orca-flash40-lt
Key Words
Wide-field | Upright microscope |
|---|
Zeiss AxioImager 2 with Apotome

Motorized upright microscope for fluorescence and transmitted light imaging with a Zeiss ApoTome for optical sectioning with structured illumination.
Nearly identical to AxioImager 1, except the ApoTome and an additional Kromnigon narrow green filter (very beneficial for samples with high levels of autofluorescence visible on the green/GFP filter).
Technical Information and capabilities
Objectives
Magnification | Objective Type | NA | Working distance (mm) | Phase-contrast | Immersion |
|---|---|---|---|---|---|
5x | EC Plan Neofluar | 0.16 | 18.5 | Ph1 | Dry |
10x | EC Plan Neofluar | 0.3 | 5.2 | Ph1 | Dry |
20x | Plan Apochromat | 0.8 | 0.55 | Ph2 | Dry |
40x | EC Plan Neofluar | 0.75 | 0.71 | - | Dry |
40x | EC Plan Neofluar | 1.3 | 0.21 | - | Oil |
63x | Plan Apochromat | 1.4 | 0.19 | - | Oil |
100x | EC Plan Neofluar | 1.3 | 0.20 | - | Oil |
Filters
Position | Filter | Excitation | Emission | Dichroic |
|---|---|---|---|---|
1 | Transmitted light | - | - | - |
2 | 49 DAPI | 365 | 445/50 | 395 |
3 | 47HE CFP | 436/25 | 480/40 | 455 |
4 | 38HE GFP | 470/40 | 525/50 | 495 |
5 | 46HE YFP | 500/25 | 535/30 | 515 |
6 | 31 Alexa 568 | 565/30 | 620/60 | 585 |
7 | 64HE mPlum | 587/25 | 647/70 | 605 |
8 | 50 Cy5 | 640/30 | 690/50 | 660 |
9 | 25HE DAPI/FITC/TxRed | Triple | Triple | Triple |
10 | Kromnigon 488 | 495/20 | 524/15 | - |
Camera
- Hamamatsu Orca Flash 4.0 LT B&W camera for fluorescence.
- Zeiss AxioCam 105 color camera for transmitted light.
Lightsource
- HXP 120 V fluorescence light source with motorized brightness adjustment and integrated shutter.
Software
- Zeiss Zen 2.
User manuals and quick instructions:
There are new Quick Start Guides available for ZEN Blue. They include the "First Steps", multidimensional imaging and import & export guides.
Some of the functions and images refer to the ZEN Lite and are different from the full version we have, which has more functionality.
There is also a video tutorial available on YouTube.
How to set up the Köhler illumination video tutorial on YouTube.
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.
https://www.fpbase.org/microscope/iSNJXmZbabCsPU8tvP9Lwf/?d=hamamatsu-orca-flash40-lt
Key Words
Widefield | Upright microscope | Optical sectioning |
|---|
Leica Thunder Imager Model Organism

A Leica M 205 FCA stereo microscope with computational clearing, motorized stage, and stage top incubator for live imaging. The THUNDER Imager Model Organism allows fast and easy 3D exploration of whole organisms for developmental or molecular biology research. It is the optimal instrument for studying, for example, Drosophila, C. elegans, zebrafish, plants, and mice.
Technical information
Apochromatic 16:1 zoom optics and 10x oculars
Objectives
- 1x PlanApo stereo objective
- 5x FluoCombi III HR objective
Light source
- Leica LED 3 fluorescence light
- Leica TL5000 transmitted light base
Fluorescence Filters
Please note that the microscope can handle 4 filters simultaneously (or 3 fluorescent filters + empty position for transmitted light). Always check which filters are installed.
Available filters:
Filter | Excitation (nm) | Emission (nm) |
|---|---|---|
DAPI | 350/50 | 460/50 |
GFP | 470/40 | 525/50 |
mCherry | 560/40 | 630/75 |
TXRed | 560/40 | 610 LP |
CY5 | 620/60 | 700/75 |
Transmitted light
- Brightfield illumination
- Rottermann contrast
- Darkfield illumination
Camera
- Leica K5 B&W sCMOS camera
Software
- Leica LAS X
- LAS X Navigator for motorized stage control
- LAS X Thunder for computational clearing
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.
https://www.fpbase.org/microscope/rH7ybAvh5EwU6E32GthEEC/
Key Words
Widefield | Stereomicroscope | Optical sectioning |
|---|
Leica Thunder Imager 3D Cell Culture
Motorized Leica DMi8 inverted epifluorescence microscope with motorized stage.

- Leica Thunder computational clearing for 3D imaging
- Fast multichannel imaging
- LAS X Navigator for multipoint imaging and large area stitching
https://www.leica-microsystems.com/products/thunder-imaging-systems/p/thunder-imager-3d-live-cell/
Technical Information
Objectives
Magnification | Objective | NA | Phase | Imm. | WD | AFC | Pixel size | Theoretical lens x-y resolution at 550nm | Theoretical lens z resolution at 550nm |
5x | N Plan | 0.12 | Ph0 | Dry | 14.0mm | - | 1.3µm | 2.796µm | 38.194µm |
10x | HC PL Fluotar | 0.32 | Ph1 | Dry | 11.1mm | + | 0.65µm | 1.048µm | 5.371µm |
20x | HC PL Fluotar Corr | 0.4 | Ph1 | Dry | 7.5mm | + | 0.325µm | 0.839µm | 3.438µm |
20x | HC PL Apo | 0.8 | - | Dry | 0.4mm | + | 0.325µm | 0.419µm | 0.859µm |
40x | HC PL Fluotar | 0.8 | PH2 | Dry | 0.4mm | + | 0.162µm | 0.419µm | 0.859µm |
63x | HC PL Fluotar | 1.1 | PH3 | IMM | 0.35mm | + | 0.103µm | 0.305µm | 0.690µm |
Filters
Please note that the filter cubes on this system are always used in combination with separate filter wheels for excitation and/or emission.
- Main filter wheel:
Quad filter cube DFT51011 (DAPI, FITC, TRITC, Cy5)
- Fast camera ex filter wheel
- 440/40nm
- 510/40nm
- 590/50nm
- 700/75nm
- 100% transmission
Camera
- Leica DFC9000GTC 4.2MPx sCMOS B&W camera.
Lightsource
- Lumencor Spectra X LED light source with wavelengths:
- 395nm, (filter 395/25)
440nm, (filter 438/29)NO CUBE- 470nm, (filter 475/28)
510nm, (filter 511/16)NO CUBE- 550nm, (filter 555/28)
- 640nm, (filter 640/30)
Okolab cage incubator
- Temperature and CO2 control
Software
- Leica LAS X 3.9.0,
3.8.2,3.7.5
Maintenance
- 40x PL APO CS2 oil removed (26.5.2023)
- LAS X software updated to 3.8.2 (11.8.2023)
- DFC9000GTC sent for service, replacement camera in use (aug- 3.11.2023)
- 63x Pl Fluotar IMM objective installed (10.11.2023)
- Okolab Cage incubator installed (10.11.2023)
- PH3 ring (for 63x Pl Fluotar IMM) installed (20.11.2023)
- LAS X software updated to 3.9.0 (20.11.2023)
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.
https://www.fpbase.org/microscope/NpZZhos5KoV2DCzG83pKE6/?c=FITC&l=spectrax-cyan&d=leica-dfc9000
Nikon Eclipse Ti-E
Fully motorized inverted wide-field microscope for fluorescence and transmitted light imaging with a full environmental chamber.

Perfect Focus System for long time series and Lumencor Spectra X light engine
The NIS-Elements software modules allow the following imaging modes:
- 2 channel ratio imaging
- Fast imaging
- High Content Screening (HCS)
Technical Information and capabilities
Objectives
Magnification | Objective type | NA | Working distance (mm) | Phase-contrast | Immersion | PFS |
|---|---|---|---|---|---|---|
4x | Plan Fluor | 0.13 | 16.5 | PhL | Dry | - |
10x | Plan Fluor | 0.30 | 16.0 | Ph1 | Dry | + |
20x | Plan Apo λ | 0.75 | 1.0 | Ph2 | Dry | + |
40x | Plan Fluor | 0.75 | 0.66 | Ph2 | Dry | + |
60x | Plan Apo λ | 1.40 | 0.13 | - | Oil | + |
100x | Plan Apo VC | 1.40 | 0.13 | - | Oil | + |
Available on request: | ||||||
40x | S Fluor | 1.30 | 0.22 | - | Oil | + |
Filters
Position | Filter | Excitation | Emission | Mirror |
|---|---|---|---|---|
1 | Quad (DAPI-GFP-TxRed-Cy5) | Quad | Quad | Quad |
2 | LED-DAPI-A | 392/23 | 447/60 | 409 |
3 | LED-FITC-A | 474/27 | 525/45 | 495 |
4 | TxRed-4040C | 562/40 | 624/40 | 593 |
5 | LED-Cy5-A | 635/18 | 680/42 | 652 |
6 | Triple (CFP-YFP-mCherry) | Triple | Triple | Triple |
Please note that the filter cubes on this system are always used in combination with separate filter wheels for excitation and/or emission. Please refer to system settings for exact combinations.
Camera
- Hamamatsu Orca Flash 4.0 V2 B&W camera for fluorescence.
Light source
- Lumencor Spectra X light engine.
- 6 solid-state light sources across the visible range
- fast wavelength switching and power adjustment
OKO Lab Bold Line Environmental Control
- OKO Lab Touch control unit has been replaced. New instructions.
Software
- NIS-Elements Advanced Research with 6D image acquisition module and JOBS high content module.
Maintenance
- New OKO Lab Touch control unit (10.2.2022)
- 40× Plan Fluor 0.75NA switched to same model with Ph2 (23.11.2022)
- Motorized stage exchanged to Nikon model (3.5.2023)
- CO2 feed calibrated (24.5.2023)
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.
https://www.fpbase.org/microscope/hdrm7drGJpj3AWZ592iJ4h/
Keywords
Widefield | Inverted microscope | High content | Live cell |
|---|
Nikon Eclipse Ti-E with Primo
Fully motorized inverted widefield microscope for fluorescence and transmitted light (DIC and phase contrast) imaging.

Equipped with long working distance objectives, motorized stage, and Nikon JOBS module for automated high content image acquisition.
Alveole Primo micropatterning device for fast multi-protein photopatterning.
Technical Information and capabilities
Objectives
Magnification | Objective type | NA | Working distance (mm) | Phase-contrast | immersion | PFS | Primo patterning compatible |
|---|---|---|---|---|---|---|---|
4x | Plan Fluor | 0.13 | 17.2 | - | Dry | - | yes |
10x | Plan Fluor | 0.30 | 16.0 | Ph1 | Dry | + |
|
20x | S Plan Fluor ELWD | 0.45 | 8.2-6.9 | - | Dry | + | yes |
40x | S Plan Fluor ELWD | 0.6 | 3.6-2.8 | Ph1 | Dry | + |
|
60x | Plan Fluor | 0.85 | 0.4-0.31 | - | Dry | - |
|
Filters
Position | Filter | Excitation | Emission | Mirror |
1 | DAPI (Semrock 5060C) | 377/50 | 447/60 | 409 |
2 | GFP (Semrock 3035D-NTE) | 472/30 | 520/35 | 495 |
3 | TxRed (Semrock 4040C) | 531/40 | 593/40 | 562 |
4 | Cy5 | 640/20 | 700/75 | 660 |
5 | Transmitted light | - | - | - |
6 | Transmitted light | - | - | - |
Camera
- Hamamatsu Orca Flash 4.0 LT B&W camera for fluorescence
Light source
- Lumencor Sola SE II 365
- Spectral coverage is from UV (DAPI excitation) to Red region (Cy5 excitation)
- Precise intensity control, fast on-off switching
Software
- NIS-Elements Advanced Research with 6D image acquisition module and JOBS high content module.
Test your own fluorophores on this optical system
To do so, click on the link below, choose the right configuration and add your own fluorophore. You will see the efficiency of the system for that specific label.