Cell counting
2D data
The best tools for quantifying the amount of cells in 2D images are ImageJ and cellprofiler. While the problem has been largely solved some care should be taken when acquiring the images. The sample should be a monolayer as cells that are on top of each other will obfuscate the results. Additionally care should be taken to not overexpose the sample as this might cause two adjacent cells to show as one during the analysis. We recommend that you take first few pictures and then analyze them and then return to the microscope and either adjust your imaging parameters or keep taking pictures if your analysis gave satisfactory results.

3D data
3D cell counting is best done on Imaris either using spots or volume tool. If your cells are spherical the spots tool is likely superior to the volume tool. If your cells come in different shapes and sizes, the volume tool is likely better. Some care should be taken when acquiring data especially in regards the resolution used. The resolution should be chosen in such a way that two distinct cells don't blur together in the final image. This can be achieved by choosing suitably small pixel size, though this might not be a feasible solution with all the samples. We recommend that you take first few pictures and then analyze them and then return to the microscope and either adjust your imaging parameters or keep taking pictures if your analysis gave satisfactory results. Cell counting methods can also be used to count any spot-like objects in the image.
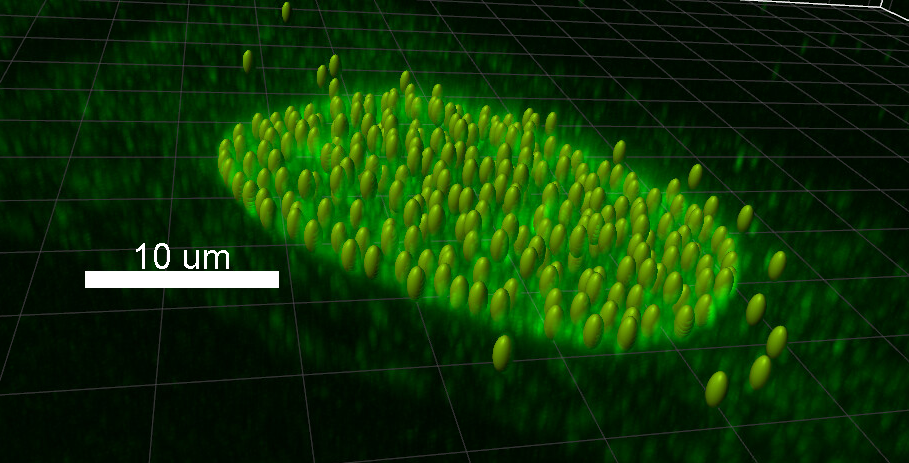
RESULTS FROM BISE
https://biii.eu/cell-or-particle-counting-and-scoring-percentage-stained-objects